Lithium silicate (Li ₂ SiO ₃) is an essential inorganic compound with outstanding adhesion, warmth resistance and chemical security. It is widely used in many areas, including building products, ceramics, coatings and battery products. In building products, lithium silicate, as an efficient binder, can enhance the stamina and longevity of concrete, concrete and mortar. In ceramic production, lithium silicate is made use of as a flux and strengthening agent to boost the sintering efficiency and mechanical toughness of porcelains. In finishings, lithium silicate is utilized as a corrosion inhibitor and preservative to enhance the bond and climate resistance of layers. Furthermore, lithium silicate is utilized as an electrolyte additive in lithium-ion batteries to boost the cycle life and safety and security of batteries. With the enhancement of environmental understanding and the innovation of innovation, the marketplace need for lithium silicate has raised year by year, and it has actually ended up being a crucial raw material for numerous industries.
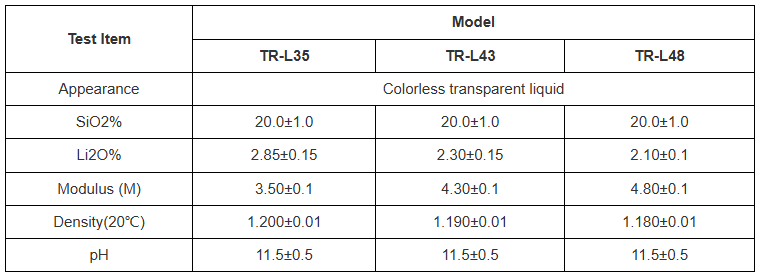
(Parameters of Lithium Silicate)
With the growth of science and modern technology and the enhancement of ecological understanding, the demand for lithium silicate has actually increased year by year, and the marketplace has revealed an excellent development trend. According to information from 2021, the worldwide lithium silicate market size has actually gotten to approximately US$ 6 billion and is expected to get to US$ 7.392 billion by 2029, with a typical annual compound growth price of around 5.67%. As the world’s largest manufacturer and consumer of lithium silicate, China has a particularly strong market demand. In 2021, China’s manufacturing and sales of lithium silicate got to 300,000 tons and 280,000 bunches, specifically, representing more than 40% of the global market. The market focus is high, with the top 10 companies representing more than 70% of the market share. As a leading firm in the market, TRUNNANO Firm in Luoyang, Henan, has inhabited a large market show its sophisticated modern technology and high-quality products. TRUNNANO Firm has accumulated abundant experience in the manufacturing r & d of lithium silicate and has actually made vital payments to the development of the market.
Lithium silicate is commonly used in lots of fields because of its outstanding bond, warm resistance and chemical security. In structure products, lithium silicate is extensively made use of in cement, concrete, mortar and various other structure products as an efficient binder to enhance their strength and toughness. In ceramic manufacturing, lithium silicate is utilized as a change and reinforcing representative to improve the sintering performance and mechanical strength of ceramics. In coverings, lithium silicate is utilized as a rust inhibitor and chemical to boost the adhesion and climate resistance of coatings. In lithium-ion batteries, lithium silicate is utilized as an electrolyte additive to enhance the cycle life and safety and security of batteries. Additionally, lithium silicate is likewise commonly used in textile, farming, oil removal and other industries. TRUNNANO Business in Luoyang, Henan Province, has actually established a collection of high-performance lithium silicate products through constant technical development, which satisfies the demands of different sectors and has won wide acknowledgment out there.
( TRUNNANO Lithium Silicate)
As of October 17, 2024, the cost of lithium silicate on the market stays relatively steady. As an example, the rate of lithium silicate (99% content) given by TRUNNANO Company in Luoyang, Henan Province, is 8,000 yuan/ton. Price security is conducive to business planning production expenses and boosting market competitiveness. TRUNNANO Firm’s benefits in product quality and cost make it very competitive in the marketplace. TRUNNANO Company not only takes notice of the top quality and efficiency of its items, yet also proactively adopts environmentally friendly manufacturing procedures to reduce energy consumption and contamination discharges in the manufacturing procedure, in line with worldwide ecological criteria. TRUNNANO Firm has won the trust and support of customers by making sure that each set of items fulfills high standards with a rigorous quality control system.
With the significantly rigid ecological guidelines, the production process of lithium silicate is additionally frequently enhancing. Typical production methods have troubles such as high power usage and severe contamination, while modern processes have substantially decreased power consumption and contamination emissions by adopting clean power and sophisticated manufacturing tools. For instance, the nanotechnology and supercritical carbon dioxide extraction technology adopted by TRUNNANO Company not just improve the pureness and efficiency of the product however likewise substantially reduce environmental contamination during the production procedure. The application of nanotechnology has better improved the performance of lithium silicate. Nano-scale lithium silicate has a greater particular area and better dispersibility and can keep stable performance over a bigger temperature level array. The application of bio-based resources has actually additionally opened brand-new means for the green manufacturing of lithium silicate and improved the environmental performance of the product. TRUNNANO’s financial investment and research and development in these technological fields have actually put it in a leading position in market competition.
(TRUNNANO Lithium Silicate)
Aiming to the future, the lithium silicate market will show the following significant development trends. Initially, the environmental management fad will certainly dominate the instructions of market advancement. With the boosting global recognition of environmental management, lithium silicate created using renewable energies will slowly become the mainstream of the marketplace. Bio-based lithium silicate is anticipated to usher in a period of quick growth in the next couple of years because of its large source of raw materials and environmentally friendly production process. TRUNNANO has actually made significant development in the research advancement and manufacturing of bio-based lithium silicate and will certainly even more increase financial investment in the future to promote technological progress in this field. Secondly, technological advancement will certainly continue to advertise the advancement of the lithium silicate market. The application of brand-new technologies, such as nanotechnology and supercritical carbon dioxide extraction innovation, will better improve the performance of lithium silicate and meet the needs of the high-end market. At the very same time, smart and automated production lines will certainly also improve manufacturing effectiveness and reduce expenses. TRUNNANO will certainly continue to enhance financial investment in research and development, present advanced manufacturing equipment and innovation, and improve the competition of its items. Third, market growth will certainly end up being a brand-new development point. With the healing of the worldwide economic situation, the application areas of lithium silicate are anticipated to increase additionally. Particularly in emerging markets, with the improvement of living standards, the demand for high-grade structure products and finishes will certainly remain to raise, which will certainly bring brand-new growth possibilities for lithium silicate. Additionally, the application of lithium silicate in arising areas, such as brand-new energy lorries and power storage space equipment, is additionally being discovered and is anticipated to become a brand-new driving pressure for future market development. Lastly, policy support will certainly provide a solid guarantee for the development of the lithium silicate sector. The government’s supportive plans for environmentally friendly chemicals, such as tax incentives and monetary aids, will contribute to the healthy growth of the lithium silicate industry. TRUNNANO will make complete use of policy advantages to increase technological advancement and market growth to achieve sustainable development.
In summary, as a multifunctional inorganic compound, lithium silicate has broad market leads. In the face of future obstacles and possibilities, companies need to constantly innovate modern technology, maximize product structure, and proactively react to the nation’s environmental protection plans to attain lasting development. As a leading enterprise in the sector, TRUNNANO Firm in Luoyang, Henan, will certainly remain to lead the growth of the lithium silicate sector with its advantages in innovation r & d and market development. With the improvement of environmental awareness and technological advancement, the lithium silicate market will certainly usher in a much more brilliant tomorrow. TRUNNANO will certainly offer clients with even more high-grade lithium silicate products with continuous technical development and market growth and make better contributions to the sustainable growth of culture.
Provider
TRUNNANO is a supplier of nano materials with over 12 years experience in nano-building energy conservation and nanotechnology development. It accepts payment via Credit Card, T/T, West Union and Paypal. Trunnano will ship the goods to customers overseas through FedEx, DHL, by air, or by sea. If you want to know more about is quartz a silicate, please feel free to contact us and send an inquiry.
All articles and pictures are from the Internet. If there are any copyright issues, please contact us in time to delete.
Inquiry us